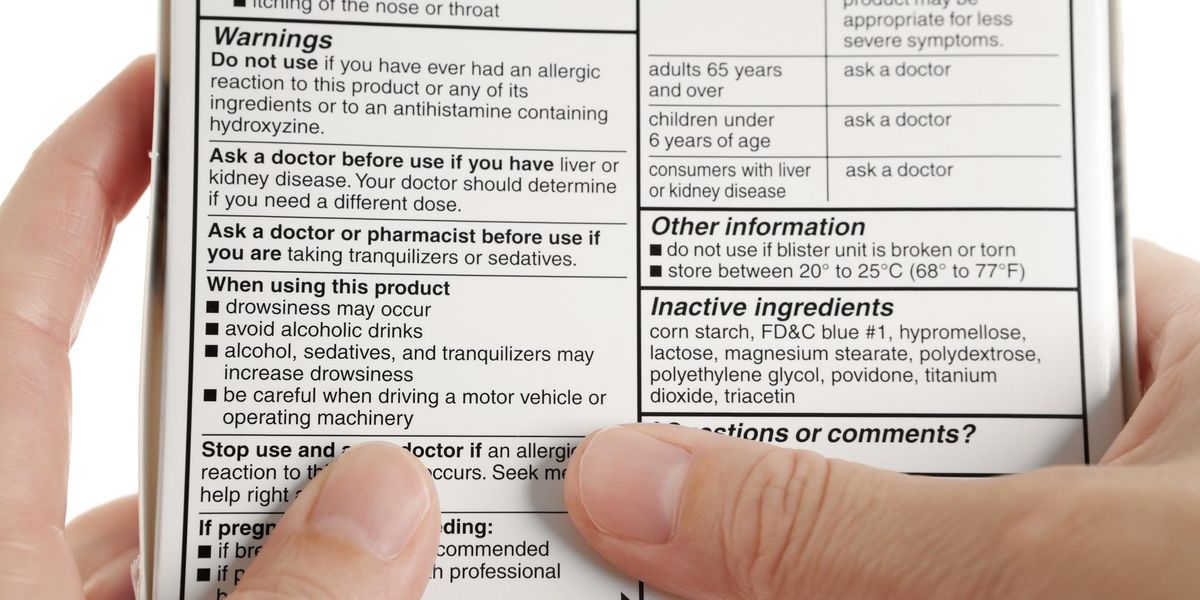
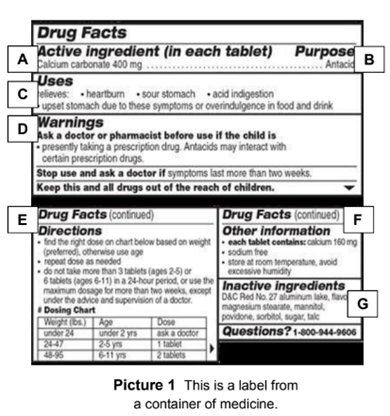
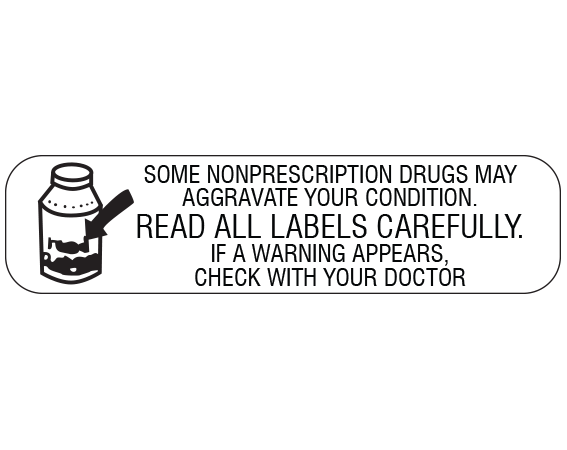
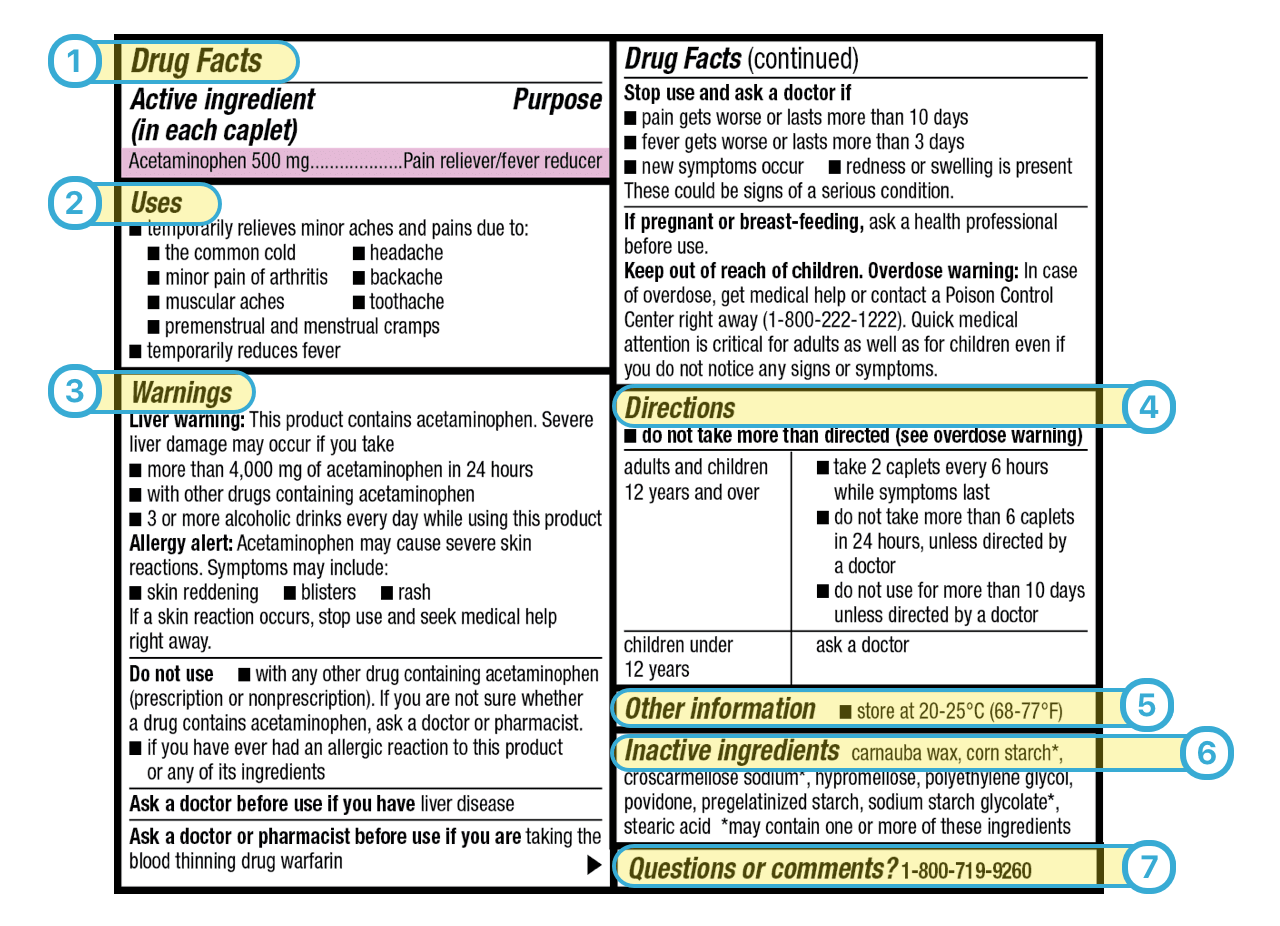
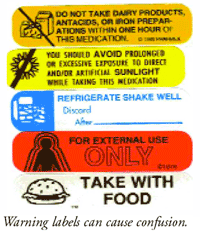
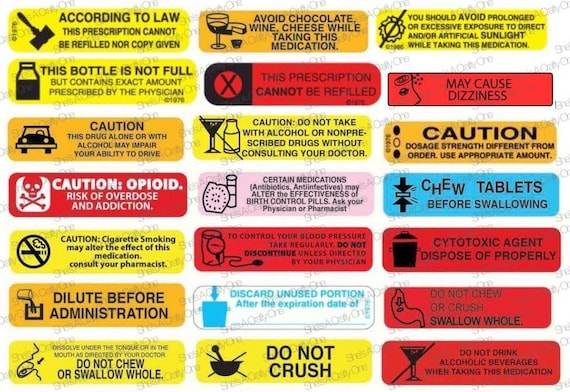
41 warning labels on drugs
Walgreens and CVS Are Under Fire for Selling This Medication to … Web03.06.2022 · She said she believed it was safe to do so as there were no warning labels or alerts from the companies about the potential risks associated with prenatal use of this medication. But when her child was 22 months old, the plaintiff said they were diagnosed with ASD. The plaintiff alleges that both Walgreens and CVS "entirely failed their duty to … 325 DOT Hazardous Materials Warning Labels and Markings - USPS WebThe warning labels shown in Exhibit 325.3 a, Exhibit 325.3 b, and Exhibit 325.4 may appear only on mailpieces containing mailable hazardous materials that require use of the label under Postal Service requirements. Division 5.1, 5.2, Class 8 and Class 9 labels are only permitted when used in conjunction with a Limited Quantity air mark.
Modafinil: Why 'smart drugs' are not the brightest option WebHowever, the Therapeutic Goods Administration (TGA) is warning consumers that off-label use of these so-called smart drugs is not a wise choice. Overseas online vendors promote drugs such as Modafinil, one of a growing category of drugs labelled nootropics , by using professional-looking and consumer friendly websites.

Warning labels on drugs
Black Box Drugs | FDA Warning Information - ConsumerSafety.org Web08.12.2017 · According to a 2006 study, nearly 40% of patients in ambulatory settings were prescribed drugs that had a boxed warning. In some of the recorded cases, pregnant women even received medications where the boxed warning made the drug contraindicated in pregnancy. With over 600 medications carrying these severe side effect warnings, it's … FDA revises labels of SGLT2 inhibitors for diabetes to include warning Web15.03.2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... FDA Drug Safety Communication: FDA strengthens warning that … WebThe U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke.
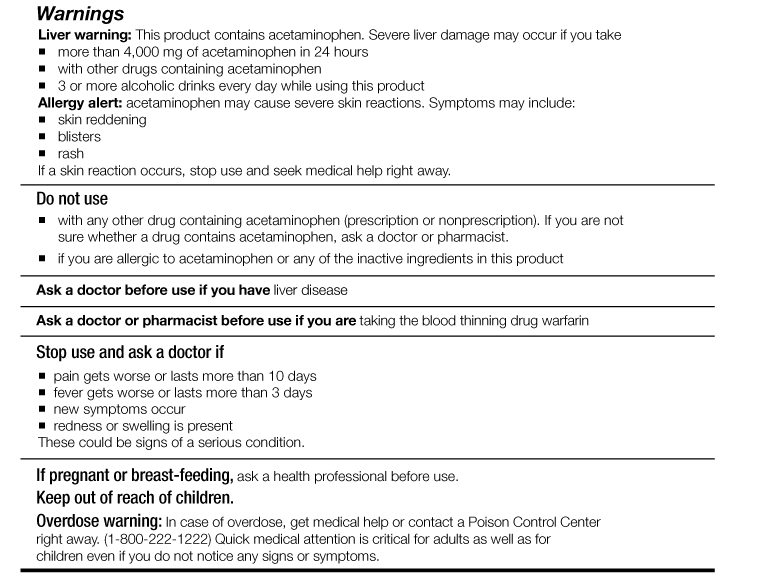
Warning labels on drugs. FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels Web23.03.2017 · FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels. 47.7K Views Modified: Jul 2, 2022 · Published: Mar 23, 2017 By Jacqueline 15 Comments. Share 9.1K. Telegram. Pin 359. 9.5K Shares. A black box warning is a consumer warning with a black border placed on labels calling out the product’s serious health risks—like the one … Academic Journals | American Marketing Association WebJournal of Marketing (JM) develops and disseminates knowledge about real-world marketing questions useful to scholars, educators, managers, policy makers, consumers, and other societal stakeholders around the world.It is the premier outlet for substantive marketing scholarship. Since its founding in 1936, JM has played a significant role in … Not a Book Ban, Yet. Florida Schools Add Book Warning Labels Web12.08.2022 · “We're talking about protecting our kids from a safety point of view, just like we don't allow guns and drugs in schools. This is no different. This is material that's age inappropriate and sexually explicit. And according to Florida statutes, should not be in public school systems,” he said. But PEN America, a nonprofit that promotes free speech, said … Boxed warning - Wikipedia WebIn the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed …
FDA Drug Safety Communication: FDA strengthens warning that … WebThe U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke. FDA revises labels of SGLT2 inhibitors for diabetes to include warning Web15.03.2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... Black Box Drugs | FDA Warning Information - ConsumerSafety.org Web08.12.2017 · According to a 2006 study, nearly 40% of patients in ambulatory settings were prescribed drugs that had a boxed warning. In some of the recorded cases, pregnant women even received medications where the boxed warning made the drug contraindicated in pregnancy. With over 600 medications carrying these severe side effect warnings, it's …

























Post a Comment for "41 warning labels on drugs"